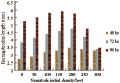Efficacy of the Steinernematid and Heterorhabditid Nematodes for Controlling the Mosquito, Culex quinquefasciatus Say (Diptera: Culicidae) 
2. Zoology and Nematology Department, Faculty of Agriculture, Cairo University, Egypt
 Author
Author  Correspondence author
Correspondence author
Journal of Mosquito Research, 2013, Vol. 3, No. 5 doi: 10.5376/jmr.2013.03.0005
Received: 12 Jan., 2013 Accepted: 23 Jan., 2013 Published: 28 Feb., 2013
Zohdy et al., 2013, Efficacy of the Steinernematid and Heterorhabditid Nematodes for Controlling the Mosquito, Culex quinquefasciatus Say (Diptera: Culicidae), Vol.3, No.5 35-46 (doi: 10.5376/jmr.2013.03.0005)
Entomopathogenic nematodes can be considered effective biocontrol agents of pest insects in aquatic habitat. Larvae of Culex quinquefsciatus Say were exposed to infective juveniles of Heterorhabditis bacteriophora, H. indica, Steinernema carpocapsae, and S. feltiae under laboratory conditions. The bioassay studies revealed the suppressive role of H. bacteriophora and H. indica nematode in controlling the mosquito, C. quinquefasciatus. They successfully established themselves in the host cadaver and produced infective juveniles. On the other hand, both S. carpocapsae and S. feltiae failed to establish in the host larvae or attain significant host mortality values. This is the first report of parasitism of entomopathogenic nematodes isolates from Egypt against larvae of C. quinquefasciatus, with promising results. Therefore, further studies must be carried out to determine if these nematodes would be effective as autochthonous agents for the control of Culex sp. and other mosquitoes of sanitary interest.
2.7 Nematode fecundity
2.8 Statistical analyses
3 Results and Analysis
A significant increase in host larval mortality was achieved by using both Heterorhabditis species, where H. bacteriophora was significantly more virulent than H. indica (P≤0.05) (Figure 1). Both Heterorhabditis species raised the host mortality levels above the normal control values by a magnitude of 2 to 4 times. In contrast, the mortality levels did not exceed 7% in case of using both Steinernema strains, with no significant difference in host mortality between both strains (P≥0.05). The host mortality levels achieved by applying both Steinernema sp. were nearly doubled when H. indica was used. In the same time, H. bacteriophora was proved to be the most virulent species, where the recorded host mortality levels were 2 times the mortality values achieved by H. indica and 3~4 times the mortality values achieved by S. feltiae and S. carpocapsae, respectively.
 Figure 1 The percentage mortality of C. quinquefasciatus larvae following exposure for 72 hr to individual ijs of H. bacteriophora (=HB), H. indica (=HI), S. carpocapsae (=SC) and S. feltiae (=SF) in the one-on-one assay. Means with the same letter are not significantly different (P≥0.05) |
When using Steinernema species, nematode individuals were melanized and were obviously seen through the host cuticle (Figure 2). In contrast, Heterorhabditis species successfully completed their life cycle within the host larvae till adult stage (Figure 3) and infective juvenile emergence. For this reason, only Heterorhabditis species were used to estimate their role in controlling the mosquito larvae.
|
|
 Figure 3 Culex quinquefasciatus larvae showing the adult stage of Heterorhabditis bacteriophora in head and thorax (showed by an arrow) |
3.2 Exposure period assay
Increasing the exposure period from 3 to 12 hr resulted in a significant increase in nematode entry to the host in both H. bacteriophora and H. indica (r=0.97, P≤0.01), (Figure 4), but the difference between numbers of H. bacteriophora juveniles and those of H. indica was not quite significant during the first 3 hr of incubation (P≥0.05). The difference between them becomes more pronounced as the duration of exposure increased from 6 to 12 hr (P≤0.01); where the number of the infective juveniles that entered the hosts was doubled.
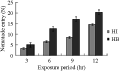 Figure 4 The effect of the exposure of C. quinquefasciatus larvae to 300 ijs of H. bacteriophora (=HB) and H. indica (=HI) per larva for different time periods on the average number of nematodes found in the insect cadaver (=N) |
Considering host mortalities, it was found that, increasing the exposure period resulted in a significant increase in host mortality (r=0.9, P≤0.01). Also, it is worthy to mention that, at all of the tested exposure periods, H. bacteriophora caused significantly higher host mortalities than those obtained by using H. indica (Figure 5). After 6 hr, the recorded host mortality values due to H. bacteriophora infection were nearly double the mortality levels recorded due to H. indica infection. By increasing the exposure time from 6 to 24 hr, the increase in host mortality became gradual in both nematode species. Maximum mortality (96.0%, 80.0%) was achieved when insects were exposed to nematodes from the species H. bacteriophora and H. indica for 48 hr, respectively (Figure 5).
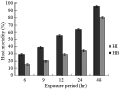 Figure 5 The effect of the exposure of C. quinquefasciatus larvae to 300 ijs of H. bacteriophora (=HB) and H. indica (=HI) per larva for different time periods on percentage host larval mortality in the exposure period assay |
Considering lethal time, it was found that H. bacteriophora was proved to be a faster killer than H. indica, where the Probit analysis showed a deduced LT90 values for H. bacteriophora of 43.12 hr in comparison with 82.64 hr for H. indica. It is worthy to mention that 12 hr of exposing the host larvae to infective juveniles of H. bacteriophora was sufficient time to kill 50% of the host population. Meanwhile, H. indica needed not less than a day to achieve the same control levels.
3.3 Dose response assay
The experiment was repeated using H. bacteriophora only and 2nd and 3rd instar larvae as host to detect the effect of host instar on the control process (Figure 7).
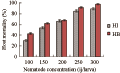 Figure 6 The percentage mortality of 4th insatr C. quinquefasciatus larvae following exposure to different concentrations of ijs of H. bacteriophora (=HB) and H. indica (=HI) |
 Figure 7 Percentage mortality of second, third, and fourth instar larvae of C. quinquefasciatus at different ij concentrateions of H. bacteriophora. Mortality was recorded after 48 hr of incubation |
Increasing nematode concentration from 100 to 300 ij/larva resulted in significantly increasing the levels of mortality in 3rd and 4th larval instars (r=0.9, P≤0.05). By using 2nd instar mosquito larvae as hosts; no significant change in larval mortality was recorded by increasing the infective juvenile concentration from 100 to 300 (P≥0.05).
The effect of increasing host instar from 2nd to 3rd instar was delayed up to 200 ij/larva, where there was no significant difference between 2nd and 3rd instar larval mortalities at lower nematode concentrations (P≥0.05). The 3rd and 4th instar larvae were more susceptible to infection than 2nd instar and the recorded host mortalities were 90.9%, 96.0% and 55 %, respectively.
Considering LC50, it was found that 121.5 ij/larva and 143.5 ij/larva were sufficient to kill 50% of 4th and 3rd instar larval populations, respectively. Meanwhile, to achieve 50% decreases in population of 2nd instar larvae, 231.8 ij/larva were needed.
Since both of the tested Heterorhabditis species completed their life cycle within the mosquito larvae, the nematode recovery was measured for both species as an indication of the ability of the nematode species to persist and multiply in this host. The infective juvenile production (recovery) for both species increased with increasing initial infective juvenile concentration up to approximately 200 infective juveniles/host (r=0.9, P≤ 0.01), where a total average of 68.3 and 97.1 infective juveniles of H. bacteriophora and H. indica, respectively, were produced (Figure 8). The maximum number of infective juveniles produced, was 136.6 ij/host for H. bacteriophora (df=14, F=37.77, P≤0.01) and 134.3 ij/host for H. indica (df=14, F= 42.25, P≤0.01). Increasing nematode concentration to more than 200 ij resulted in a decline in nematode recovery (r=-0.9, P≤0.01, Figure 8). However, the number of infective juveniles produced per host dropped to levels comparable with low initial densities when nematode initial densities were raised to 300.
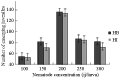 Figure 8 Mean number of individual infective juveniles of H. bacteriophora (=HB) and H. indica (=HI) emerged from a C. quinquefasciatus cadaver as a function of changing initial nematode density |
Generally, both species were similar in their recovery from cadavers as they were both interacting with the same manner to increasing nematode concentration/host with no significant difference between numbers of their produced juveniles (P≥0.05).
The effect of increasing the initial nematode concentration of H. bacteriophora on the length of the resulted hermaphrodites (produced at the first generation within the host) was determined. Since both species showed similar recovery pattern, only H. bacteriophora was selected in this assay. Changing concentration of nematodes/host resulted in a significant change in hermaphrodite length after 48,72, and 96 hr of exposure. Adult length increased with increasing initial nematode concentration up to 150 infective juvenile/host then declined with further increase in nematode concentration, P≤ 0.05 (Figure 9).
Initially, after 48 hr of exposure, only hermaphrodites at low densities (below 100) were small. The effect of high density on hermaphrodite length was delayed till 72 and 96 hr. The growth rate was nearly constant during the 1st 2 days and did not change by changing initial nematode concentration, i.e. it is not affected by crowding yet. Further increase in concentration did not negatively affect the adult length after 2 days; i.e. the nematodes can tolerate this level of crowding. After 48 hr, the adult length (3.3 mm) was still greater than those of control (2.76 mm) even at maximum nematode density of 300. The maximum adult length was 3.4 mm at nematode concentration of 200.
The effect of crowding was obvious after 3 to 4 days post infection, where the adult length changed significantly upon increasing nematode concentration from 0 to 250 and decreased to a size smaller than that of control at 300 nematode/host. The maximum adult length was 4.7 mm and 5.9 mm when using 200 and 150 infective juvenile/ host after 72 and 96 hr, respectively.
After 3 days of infection, adults were significantly shorter when concentration of nematodes infective juveniles was raised to 250 and 300. Raising the concentration from 50 up to 200 resulted in a gradual increase in adult length reaching maximum length at 150~200 nematodes/host, after which adult length decreased sharply.
After 72 and 96 hr, further increase in initial nematode density resulted in a significant decrease in hermaphrodite length comparable to control levels after 72 hr (3.7 and 3.4, respectively) and less than control after 96 hr (3.4 and 5.3, respectively) (r=-0.9, P≤ 0.01).
Increasing initial nematode concentration up to 300 infective juvenile/host resulted in a decrease in the length of the emerging juveniles of the 1st generation that were migrating out of the cadaver. Maximum infective juvenile length (651.6 µm) for H. bacteriophora occurred at low densities (50 nematode/host), not at the densities producing the maximum number of infective juveniles (Figure 10). Infective juvenile length declined sharply upon treatment until initial densities of 200 for, approximately 546.6 µm (r=-0.97, P≤0.01). The decline in length was more gradual at higher initial densities ranging from 250 to 300 nematode/host (P≥0.05).
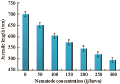 Figure 10 Mean length of individual infective juveniles of H. bacteriophora emerged from a C. quinquefasciatus cadaver as a function of changing initial nematode density |
4 Discussion
Mosquito insecticide-resistance at larval stages is extremely high compared to adult stage. For this reason, another biological, safe, and effective control method is favored over the chemical one. In the present study, the nematode impact in biological control of mosquitoes was ranked according to various factors affecting the infection process. The exposure period assay indicated, indirectly, how quickly insects were infected by the nematodes. The one-on-one and dose response assays measured the overall infection process in regards to nematode species and doses which affects the control levels as well as the nematode characteristics as fecundity.
In the present study, one-on-one assay indicated that H. bacteriophora is more virulent to mosquito larvae than H. indica and the recorded mortality values give a promising spot light on the possibility of using both species to control mosquito larvae. Our results were in agreement with the early work of Poinar & Kaul (1982), where they investigated the parasitism of C. pipiens by the nematode, H. bacteriophora and they recorded high levels of host mortality.
On the other hand, both tested Steinernema species failed to establish in the C. quinquefasciatus mosquito larvae and were melanized. Bronskill (1962) recorded melanization of Neoaplictanid rhabditoid nematode (DD136) by A. aegypti larvae.
In the exposure period assay, both Heterorhabditis species entered the host with nearly the same rate during the first 3 hr. Increasing exposure time separated the efficiencies of both species as fast invader (H. bacteriophora) and slower invader (H. indica). By the end of 12 hr exposure period, H. bacteriophora was proved to be still more active than those of H. indica and this might be the reason for the higher H. bacteriophora numbers that entered the larvae (20 ij) in comparison with (14 ij) in case of H. indica. These two parameters (invasion rate and nematode numbers) are closely related, and are considered characteristic for specific nematode species. On the other hand, Abd El Rahman & Hussein (2007) recorded that H. indica invaded G. mellonella larvae in higher numbers than H. bacteriophora only at lower initial nematode concentration while H. bacteriophora entered in higher numbers than H. indica at high nematode concentrations.
Concerning the effect of exposure period on host mortality, H. bacteriophora was found to be a faster killer for C. quinquefasciatus (with high invasion rate) than H. indica. This was clear from the LT90 values. In this context, H. bacteriophora needed 43.12 hr to kill 90% of the host population which is nearly half of the time needed by H. indica to attain the same host mortality values. These results support the previous ones concerning nematode entrance as a measure of invasion rate. However, low penetration level and slow invasion rate may not necessarily mean that a nematode has lower efficacy. In the present work, H. indica raised the host mortality percentages very efficiently reaching 80%, which is a good indication of virulence towards this host species. This contrast may be attributed to variation in infection strategies. Since the infective juveniles of heterorhabditid nematodes develop into hermaphrodites, a single invader can potentially reproduce. For this reason, low number of nematodes entering to their hosts will be sufficient to establish the next generation. In contrast, steinernematids are amphimictic and mating is necessary to reproduce, thus an invasion of high numbers of individuals increases the probability of mating and further reproduction (Koltai et. al., 1995).
The data obtained in the exposure period assay suggest that this assay may be used to compare different species or production batches of nematodes with different penetration ability. However, the biological impact of this assay and its relationship to nematode activity in the field is yet to be determined.
The dose response assay could be another way to determine nematode virulence. The increase in nematode infective juvenile numbers/ host larva resulted in an increase in mortality of 3rd and 4th instars of mosquito larvae after using both Heterorhabditis nematode species. These results were in agreement with the effect of host size recorded by (Poinar & Kaul, 1982). From the factors that governed the degree of infection is the stage of the host. Parasitism in general was highest in fourth instar larvae. This is due to the fact that larger hosts could more readily ingest nematodes without damaging them. In contrast, second-instar larvae rarely ingested whole nematodes, more often crushing them with their mandibular teeth because of their smaller oral aperture. Once the nematode cuticle was broken, the parasite perished.
Our results and early work of Dadd (1971) and Poinar & Kaul (1982) were following the same pattern of response to changing nematode concentration, but their work showed the inability of the rhabditoid nematode-bacteria system to complete their life cycle in C. pipiens mosquitoes. However, the mortality records were in agreement with our records concerning the host instar effect. They observed that although 4th instar larvae may ingest hundreds of nematodes within few hours, very few individual successfully established themselves in the hemocoel and that those worms remaining within the peritrophic membrane commence to disintegrate within a few hours, often leaving no recognizable remains after a day. But the benefit of our nematode strains is that they were able to complete their life cycle in their host larvae till infective juvenile emergence. The same pattern of dose response was found in their experiment.
These results were in agreement with those of Capinera et al., (1988) who found a positive correlation between numbers of infective juvenile used and the number of nematodes which successfully invaded the host. Meanwhile, Abd El Rahman & Hussein (2007) did not found a significant effect of increasing nematode concentration from 250 to 500 ij/host neither on mortality of L. decemlineata larvae nor on the nematode invasion rate. They also did not find a significant difference in 3rd and 4th instar larval mortality upon exposure to H. bacteriophora infective juveniles for 24 to 72 hr.
Considering nematode fecundity after changing initial nematode concentration per host, the results showed that H. bacteriophora was able to tolerate high densities within the host than H. indica but both species responded with the same pattern to increasing nematode concentration. Metabolic rate, processing of host tissues by symbiotic bacteria, and the physiological parameters required for growth differ between nematode species. Differences between both heterorhabditid species may result from the differences in host utilization. Also, the reason for the differences in the effect of viable bacterial cells of P. luminiscens (H. bacteriophora symbionts) may be that these bacteria could survive more the host immune response and present toxic components that killed the host while the bacterial symbionts of H. indica were susceptible to host immune system (Selvan & Blackshaw, 1990).
The effect of initial infection density was noticed clearly in the quality of the produced nematode adults as well as the produced juveniles which in turns had an important influence on the population dynamics of parasites. Hermaphrodite length is often used as an indirect measure of nematode fecundity (Selvan et al., 1993). The decline in length which is an indication of reduced fecundity at low density may be probably due to either the decreased level of bacterial inoculums causing slower break down of host tissues, or conversely, insufficient bacterial culling by the nematode resulting in unrestrained bacterial growth. In contrast, at high densities, host utilization by nematode and bacteria is more rapid and may result in inadequate nutrition resulting from competition for limited nutrients within the host. Because H. bacteriophora was able to survive at high densities, this could explain how it successfully reproduced at all of the studied densities.
The effect of the initial infection density on juvenile length was clearly obvious, where the longest infective juveniles were produced at lowest densities, not at the densities producing the largest number of infective juveniles. Abd El Rahman & Hussein (2007) recorded a negative correlation between infective juvenile length of both H. bacteriophora and H. indica with increasing infection density. In this respect, the larger host supports more the development of higher numbers of juveniles without competitions and constrains that were found in small hosts, where crowding effect appears as a great factor affecting juvenile growth and hence juvenile length.
The tradeoff between quantity and quality of emerging infective juveniles (in respect to length) has been reported early for other parasites (Kino, 1984). Because taller infective juveniles harbor more nutrients, they can be expected to survive for a longer period than shorter nematodes. These juveniles were expected to be more active and hence have higher searching capacity than shorter, less active ones. In contrast, producing large numbers of small, short-lived infective juveniles may decrease the probability of rapidly locating a new host. The explanation of these results may rely on the lipid content of the nematode juveniles. Lewis et al. (1995) studied the relationship between the metabolic rate, energy reserves, and foraging behavior in three species of entomopathogenic nematodes; S. carpocapsae, S. glaseri, and H. bacteriophora, each species is characterized by differing in juvenile length. Their studies showed that lipids, the major components of nematode energy reserves, were stored in larger quantities in longer juveniles than in shorter ones. These lipids were declined at species-specific rates.
The density-dependent factors may play an important role in entomopathogenic nematode fecundity. The density dependent effect may be important in regulating nematode populations either by acting directly through affecting the numbers of infective juveniles produced from each cadaver, or indirectly, by changing the infective juvenile longevity. In laboratory culturing and biological control applications where recycling and persistence is advantageous, the impact of infection density may be of critical importance in maximizing nematode efficacy.
The dose response bioassays has been used many times previously and probit analysis has been used to analyze the data to calculate LC50 values. However, when a parasite is highly virulent, the applicability of probit analysis is questionable, since a single steinernematid or heterorhabditid nematode is often capable of killing an insect (Ricci et al., 1996).
Although similar ranking was observed in the present bioassays, the ability to separate the species statistically varied among assays. One-on-one assay effectively separated H. bacteriophora and H. Indica from each other and from S. carpocapsae and S. feltiae. The later two species could not be separated from each other by this assay. This assay was conducted in multi-well plates, so, nematodes and insects were kept in close contact and the influence of foraging strategies was limited. Differences in nematode ability to penetrate into the insect and complete its life cycle served as the main factor distinguishing between species. Also, the dose response assay could not separate both heterorhabditid species from each other except in the low and high doses of 100 and 200 ij/insect after 48 hr. These treatments were the best for separating both species. The LC50 or LC90 values also separated both species.
The present work dealt with demonstrating the variations of entomopathogenic nematode species performance in different bioassays. The differences in the activity of nematodes in the exposure period assay made a spot light on the potential of measuring some behavioral responses as specific criteria for nematode virulence. The presented data support the fact that, since nematodes vary in their behavior, one bioassay cannot be used as a unique measure of virulence for all species (Caroli et al., 1996).
In general, particular bioassays may be used for other purposes: for the selection of a specific population for use against an insect, a variable assay measures which are more laborious but simulate natural environmental conditions or invasion by nematode (e.g. nematode entrance) should be considered. In cases where production batches of the same nematode strain are compared, a simple rapid assay is needed (e.g. one-on-one or exposure period assay). The obtained results may add much to our information concerning the use of nematode-bacteria system to control C. quinquefasciatus larvae. Its significance is that it is the first attempt in Egypt to get benefits of augmenting host-specific, lethal bacteria within the nematode to the aquatic larvae to reduce the mosquito population before adult emergence as well as producing nematode progeny that can re-infect another bottom-feeding host nearby in the system.
The use of laboratory pathogenecity bioassays in these experiments has been relevant in showing consistently the pathogenic capability of the heterorhabditid nematodes over steinernematid one and hence their symbiotic bacteria, P. luminiscens over that of X. nematophilus to C. quinquefasciatus larvae, although, these laboratory bioassays do not provide an assurance of field efficacy, so, field application studies should be done. However, they guaranteed the promising value of using entomopathogenic nematodes of the genus Heterorhabditis in controlling the aquatic larvae. These aquatic systems do not introduce the detrimental effect of sun light and ultraviolet radiation as well as desiccation on these types of nematodes as it usually happens in agricultural systems which are considered the most important factors that lower the efficacy of applying these entomopathogenic nematodes in agricultural systems. This was because the bacteria are susceptible to sunlight and U.V. radiation, also, desiccation is a major concern for the nematode itself, where all nematodes need at least a water film to move through it and infect a host. In this system of aquatic habitat, many of the mosquito larvae are bottom feeders, and hence they were protected from sunlight and U.V. radiation.
References
Abbott W.S., 1925, A method of computing the effectiveness of an insecticide, J. Econ. Entomol., 18: 265–267
Abd El-Rahman R.M., and Hussein M.A., 2007, Effect of different infection rates in Galleria mellonella larvae on the quality of the produced Heterorhabditis juveniles, Egypt. J. Biol. Pest Control, 17(2): 91-97
Achinelly M.F., Micieli M.V., Marti G.A., and García J.J., 2004, Susceptibility of neotropical mosquito larvae (Diptera: Culicidae) and non-target aquatic organisms to the entomoparasitic nematode Strelkovimermis spiculatus Poinar & Camino, 1986 (Nematoda: Mermithidae), Nematology, 6: 299-302
http://dx.doi.org/10.1163/1568541041217951
Bedding R.A., and Akhurst R.J., 1975, A simple technique for the detection of insect parasitic rhabditid nematodes in soil, Nematologica, 21:109-110
http://dx.doi.org/10.1163/187529275X00419
Begley W., 1990, Efficacy against insects in habitats other than sail, ln: Gaugler R. and Kaya H.K., Eds, Entomopathogenic nematodes in biological control, Boca Raton, FL, USA, CRC Press: 215-231
Boemare N., 2002, Entomopathogenic nematology, Wallingford, UK: CABI Publishing, Biology, taxonomy and systematics of Photorhabdus Xenorhabdus, P: 35-56
http://dx.doi.org/10.1079/9780851995670.0035
Bronskill J.F., 1962, Encapsulation of Rhabditoid nematodes in mosquitoes, Can. J. Zool., 40: 1269-1275
http://dx.doi.org/10.1139/z62-103
Burnell A.M., and Stock S.P., 2000, Heterorhabditis, Steinernema and their symbionts lethal pathogens of insects, Nematologica, 2: 31-42
http://dx.doi.org/10.1163/156854100508872
Cagnolo S.R., and Almirón W.R., 2010, Capacity of the terrestrial entomopathogenic nematode Steinernema rarum (Rhabditida: Steinernematidae) to parasite Culex apicinus larvae (Diptera: Culicidae), Rev. Soc. Entomol. Argent., 69 : 141-145
Capinera J.L., Pelissier D., Menout G.S., and Epsky N.D., 1988, Control of black cutworm, Agrotis ipsilon (Lepidoptera: Noctuidae), with entomogenous nematodes (Nematoda: Steinernematidae, Heterorhabditidae), J. Invert. Pathol., 52: 427-435
http://dx.doi.org/10.1016/0022-2011(88)90055-9
Caroli I., Glazer I., and Gaugler R., 1996, Entomopathogenic nematode infectivity assay: Comparison of penetration rate into different hosts, Biocontrol Science and Technol., 6:227-233
http://dx.doi.org/10.1080/09583159650039412
Dadd R.H., 1971, Size limitations on the infectibility of mosquito larvae by nematodes during filter-feeding, J. Invert. Pathol., 18: 246-251
http://dx.doi.org/10.1016/0022-2011(71)90152-2
de Doucet M.M.A., Miranda M.B., and Bertolotti M.A., 1998, Infectivity of entomogenous nematodes (Steinernematidae and Heterorhabditidae) to Pediculus humanus capitis De Geer (Anoplura: Pediculidae), Fundam. Appl. Nematol., 21: 13-16
Finney D.J., 1971, Probit Analysis (3rd. ed.), London Cambridge University Press, 197: 318
Friedman M.J., 1990, Commercial production and development, In: Entomopathogenic Nematodes in Biological Control, Gaugler R., and Kaya H.K., Eds., CRC Press, Boca Raton, FL., pp.153-172
Grewal P.S., and Georgis R., 1998, Entomopathogenic nematodes, In: Methods in Biotechnology, Hall F.R., and Menn J., Eds., Biopesticides: Use and Delivery, Humana Press, Totowa, 5: 271-299
Grewal P.S., Ehless R.U., and Shapiro-Ilan D.I., 2005, Nematode as biological control agents, Wallingford, UK: CABI Publishing, pp:115-146
http://dx.doi.org/10.1079/9780851990170.0000 http://dx.doi.org/10.1079/9780851990170.0115
Hazir S., Kaya H.K., Stock S., and Kesk P.N., 2004, Entomopathogenic Nematodes (Steinernematidae and Heterorhabditidae) for Biological Control of Soil Pests, Turk. J. Biol., 27: 181-202.
Kaya H.K., and Gaugler R., 1993, Entomopathogenic nematodes, Ann. Rev. Entomol., 38: 181-206
http://dx.doi.org/10.1146/annurev.ento.38.1.181 http://dx.doi.org/10.1146/annurev.en.38.010193.001145
Kino H., 1984, Parasite density and the fecundity of Angiostrongylus cantonensis in rats, Parasitology, 89: 275-286
http://dx.doi.org/10.1017/S003118200000130X PMid:6504559
Koltai H., Glazer I., and Segal D., 1995, Reproduction of the entomopathogenic nematode Heterorhabditis bacteriophora Poinar: Hermaphroditism vs Amphimixis, Fundam. Appl. Nematol, 18: 55-61
Lewis E.E., Selvan S., Campbel J.F., Gaugler R., 1995, Changes in foraging behaviour during the infectctive juvenile stage of epn, Parasitol., 110: 583-590
http://dx.doi.org/10.1017/S0031182000065306
Miller R.W., 1989, Novel pathogenicity assessment technique for Steinernema and Heterorhabditis entomopathogenic nematodes, J. Nematol., 21: 574
Park Y., Kim Y., Tunaz H., and Stanley D.W., 2004, An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits haemocytic phospholipase A2 (PLA2) in tobacco hornworms, Manduca sexta, J. Invert. Pathol., 86: 65-71
http://dx.doi.org/10.1016/j.jip.2004.05.002 PMid:15261769
Pérez-Pacheco R., Rodríguez-Hernández C., Lara-Reyna, J., Montes-Belmont R., Ramírez-Valverde G., and Martínez-Martínez L., 2004, Parasitismo de Romanomermis iyengari en larvas de tres especies de mosquitos en laboratorio y de Anopheles pseudopunctipennis en campo, Agrociencia, 38(4): 413-421
Poinar G.O.Jr., and Kaul H.N., 1982, Parasitism of the mosquito Culex pipiens by the nematode Heterorhabditis bacteriophora, J. Invert. Pathol., 39: 382-387
http://dx.doi.org/10.1016/0022-2011(82)90063-5
Poinar G.O.Jr., 1989, Non-insects hosts for the entomogenous rhabditoid nematodes Neoaplectana (Steiner-nematidae) and Heterorhabditis (Heterorhabditidae), Rev. Nematol., 12: 423-428
Poinar G.O.Jr., 1990, Taxonomy and biology of Steinernematidae and Heterorhabditidae, In: Entomopathogenic nematodes in biological control, Gaugler R., and Kaya H.K., Eds., Boca Raton, FL: CRC Press., pp: 23-61
Ricci M., Glazer I., Campbell J.F., and Gaugler R., 1996, Comparison of Bioassays to Measure Virulence of Different Entomopathogenic Nematodes, Biocontrol Science and Technol., 6: 235-246
http://dx.doi.org/10.1080/09583159650039421
Santamarina M.A., Pérez-Pacheco R., and Martínez S.H., 2000, Susceptibilidad de las larvas de Aedes aegypti al parasitismo por Romanomermis culicivorax en condiciones de laboratorio y de campo en Oaxaca, México. Rev. Panam. Salud Pública, 8: 299-304
http://dx.doi.org/10.1590/S1020-49892000001000001
Selvan S., and Blackshaw R.P., 1990, The influence of Neoaplectana bibionis and Heterorhabditis heliothidis and their associated bacteria on oxygen consumption in Galleria mellonella, J. Invert. Pathol., 56: 20-24
http://dx.doi.org/10.1016/0022-2011(90)90139-W
Selvan S., Gaugler R., and Lewis E.E., 1993, Biochemical energy reserves of entomopathogenic nematodes, J. Parasitol., 79: 167-172
http://dx.doi.org/10.2307/3283503
Selvan M.S., and Muthukrishnan J., 1988, On certain correlations between host parameters and parasitoid production, Ann. Entomol., 8: 1-10
Silverman J., Platzer E.G., and Rust M.K., 1982, Infection of the cat flea, Ctenocephalides felis (Bouché) by Neoapleetana carpocapsae (Weiser), J. Nematol., 14: 394-397
PMid:19295728 PMCid:2618202
Wang J., and Bedding R.A., 1996, Population development of Heterorhabditis bacteriophora and Steinernema carpocapsae in the larvae of Galleria mellonella, Fund. Appl. Nematol., 19: 363-367
Weiss M., Glazer I., Mumcuoglu K.Y., Elking Y., and Galun R., 1993, Infectivity of steinernematid and hererorhabditid nemarodes for the human body louse Pediculus humanus humanus (Anoplura: Pediculidae), Fundam. Appl. Nematol., 16: 489-493
Welch H.E., and Bronskill J.F., 1962, Parasitism of mosquito larvae by the nematode DD136 (Nematoda: Neoaplictanidae), Can. J. Zool., 40: 1200-1208
http://dx.doi.org/10.1139/z62-102
Woodring J.L., and Kaya H.K., 1988, Steinernematid and heterorhabditid nematodes: A hand book of biology techniques, Ark. Agric. Exp. Stn. South Coop. Serv. Bull., 331, pp. 1-30
Zhioua E., Lebrun R.A., Glnaberg H.S., and Aeschumat N.A., 1995, Pathogenicity of Steinernema carpocapsae and S. glaseri (Nemaroda: Steinernematidae) to Ixodes scapularis (Acari: Ixodidae), J. med. Eni., 32: 900-905
. PDF(292KB)
. FPDF
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Nawal Zohdy
. Muhammed Shamseldean
. Emtithal Abd El-Samiee
. Heba Mohammed Hamama
Related articles
. Mosquito
. Culex quinquefsciatus
. Nematode
. Heterorhabditis bacteriophora
. H. indica
. Steinernema carpocapsae
. S. feltiae
. Bioassay
Tools
. Email to a friend
. Post a comment